The electrons occupy fixed positions around the protons which are at the center of the atom. According to Rutherford model of the atom.

The Quantum Mechanical Model Of The Atom
The quantum mechanical model of the atom uses complex shapes of orbitals sometimes called electron clouds volumes of space in which there is likely to be an electron.
. The electrons move around the protons which are at the center of the atom. According to the Bohrs atomic model the electrons revolve around the nucleus in a fixed orbit. Thinking about electrons as probabilistic matter waves using the de Broglie wavelength the Schrödinger equation and the Heisenberg uncertainty principle.
A It is a spherical or dumbbell-shaped route traced by the electron in its rapid movement. Chemistry questions and answers. Eliminate b has discrete energy levels.
4 An atom is electrically neutral. Which statement correctly describes the quantum number n with reference to the Bohr model of the hydrogen atom. What is quantum mechanical description of an atomThe quantum mechanical model is based on quantum theory which says matter also has properties associated with waves.
In Bohrs model of the atom where are the electrons and protons located. Learn vocabulary terms and more with flashcards games and other study tools. C It is a region in space in.
Which statement describes only the quantum mechanical model of the atom. A energy is quantized. Due to this high probability orbits are replaced by the term orbital.
The electrons and protons move throughout the atom. B It is a region in space that has a precise shape and is completely filled hva dense electron cloud. Start studying Chapter 8.
The quantum mechanical model of the atom Introduction to the quantum mechanical model of the atom. Which statement best describes the quantum mechanical modern model of the atom. The Quantum-Mechanical Model of the Atom.
An electron with n 2 is at higher energy than an electron with n 1. 2 Major space in an atom is empty. According to the new current Quantum atomic model An orbital is a region of space in an atom where the.
Select all that apply. Which statement best describes an occupied orbital according to the quantum mechanical model. An atom consists of a central nucleus with proton neutrons and electrons orbiting in levels of high probability.
D sometimes referred to as the planetary model. 3 Atoms nucleus is surrounded by negatively charged particles called electrons. C has sub levels within the energy levels.
The majority of the mass of an atom is outside of the nucleus. 1 Atoms have their charge concentrated in a very small nucleus. Greek philosopher Democritus coined the term atom.
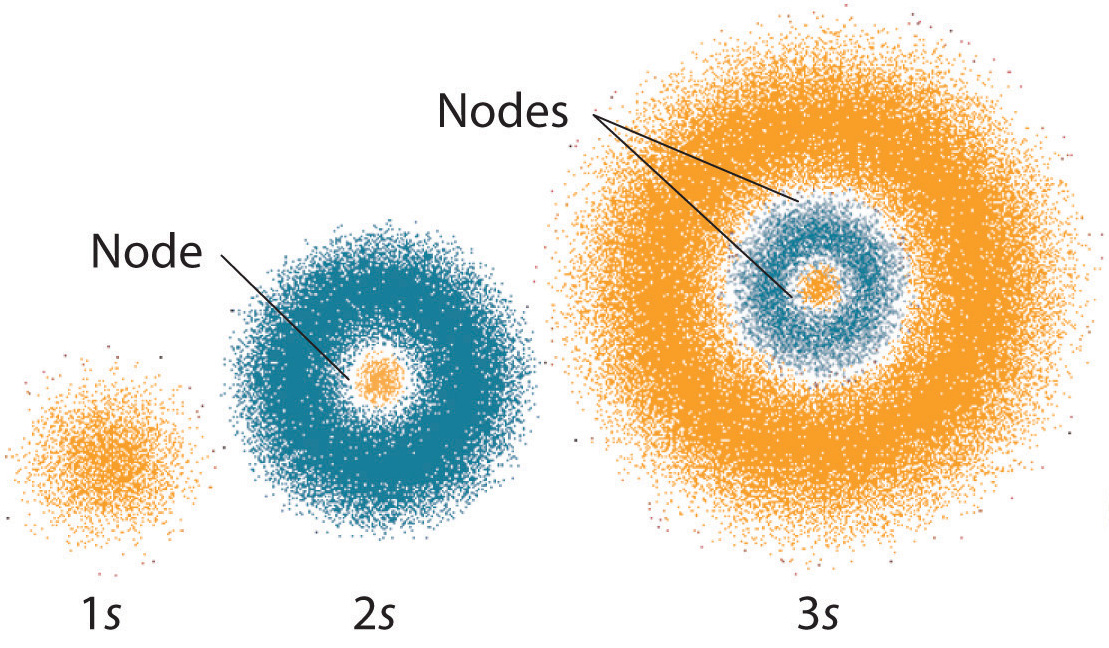
The Quantum Mechanical Model Of The Atom Article Khan Academy

Sound Really Is At The Foundation Of The Structure Of Matter Itself Learn More Http So Meditation Methods Personal Development Blog Posts Quantum Mechanics
0 Comments